Introduction: Brentuximab vendotin (BV) with AVE-PC demonstrated superior efficacy to ABVE-PC for pediatric patients with high-risk HL in the AHOD 1331 trial. However, there are no data regarding the impact on health-related quality of life (HRQoL) with the addition of BV into the treatment of pediatric HL.
Methods: Eligibility for AHOD 1331 (NCT 02166463) included previously untreated HL with high risk disease, defined as stage IIB +bulk, IIIB, IVA, or IVB classic HL. Participants were randomized to either BV-AVE-PC (“BV” arm) or ABVE-PC (standard arm). Among the first 309 participants enrolled in a prespecified longitudinal patient-reported outcomes sub study, 280 were age 11+ years and eligible to self-report HRQoL using the seven-item Child Health Ratings Inventories (CHRIs)-Global scale. HRQoL was assessed prior to treatment (T1), at cycle 2 (T2), at cycle 5 (T3), and at the end of treatment (T4). A clinically meaningful increase in HRQoL was considered a change of 7 points in the CHRIs-Global score (CHRIs), translating to a 1/3 standard deviation. Multivariable linear regression estimated the association between demographic/clinical variables and HRQoL at T1. Linear mixed models estimated the change in HRQoL relative to T1 with a time*treatment arm interaction to estimate differences in the change in HRQoL across treatment arm. This model was adjusted for age, sex, race/ethnicity, stage, large mediastinal adenopathy (LMA), B-symptoms at diagnosis, and a time-varying covariate for involved site radiation prior to T4.
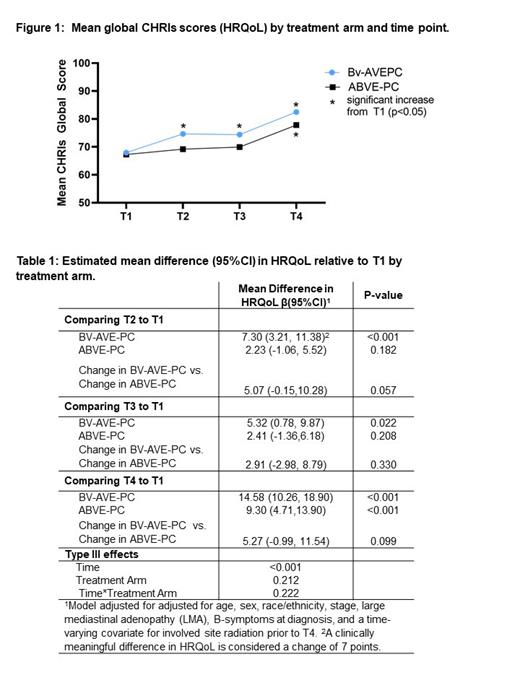
Results: Participant and disease characteristics were balanced by treatment arm; mean age at enrollment was 15.6 years (SD= 1.9), 52% were female, 60.4% had LMA, and 58% were stage IV. 93% of participants completed the CHRIs at T1, 92% at T2, 89% at T3, and 74% at T4. At T1, female sex (mean (SD) 64.1 (20.2) vs 71.3 (23.3) p=0.004) and fever (60.8 (23.0) vs. 71.9 (20.4) p=0.024) were associated with worse HRQoL. At each time point after pre-treatment (T1), HRQoL was higher, on average, in the BV arm compared to the standard arm (Figure 1). By T2, participants in the BV arm experienced a statistically and clinically significant increase in HRQoL compared to pre-treatment (T1) (Table 1; β=7.3 95%CI 3.2, 11.4; p≤0.001), which was greater than the change experienced in the standard arm (difference in change β=5.1 95%CI -0.2, 10.3; p=0.057). At T3 and T4 the BV arm continued to experience statistically significant improvements in HRQoL relative to T1 (T3 β=5.3 95%CI 0.8, 9.9; p=0.022; T4 β=14.6 95%CI 10.3, 18.9; p≤0.001). The standard arm did not experience a statistically or clinically significant increase in HRQoL from T1 until T4 (β=9.3 95%CI 4.7, 11.5; p<0.001).
Conclusion: Patients with high-risk pediatric HL treated with BV-AVE-PC experienced more rapid and sustained improvement in HRQoL compared to patients treated with ABVE-PC. These data also demonstrate the feasibility and importance of incorporating HRQoL assessments into pediatric clinical trials.
Disclosures
Keller:Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific advisory council. Kelly:Seagen: Other: Scientific Steering Committee; Merck: Other: Scientific Steering Committee. Castellino:Bristol Meyers Squibb: Honoraria, Other: Scientific Advisory Committee; SeaGen Inc.: Other: Scientific Advisory Committee - No honoraria, Research Funding. Parsons:Seagen: Consultancy.